Does Zinc Oxidize?
Absolutely, zinc does oxidize. In fact, oxidation is a fundamental chemical reaction that happens to most metals, including zinc. Let’s break this down to understand it better.
Understanding Oxidation
Before diving into zinc specifically, you should know what oxidation is. Oxidation involves a chemical reaction where a substance loses electrons. When talking about metals, this usually means reacting with oxygen in the air.
How Zinc Oxidizes
When zinc comes into contact with oxygen, it undergoes a chemical reaction. This process results in the formation of zinc oxide. This is not just a theory; it’s a well-known and widely observed phenomenon in chemistry.
The Process
- Initial Reaction: Zinc (Zn) reacts with oxygen (O2) in the air.
- Formation of Zinc Oxide: The reaction produces zinc oxide (ZnO).
This is represented by the chemical equation: 2 Zn + O2 →2 ZnO
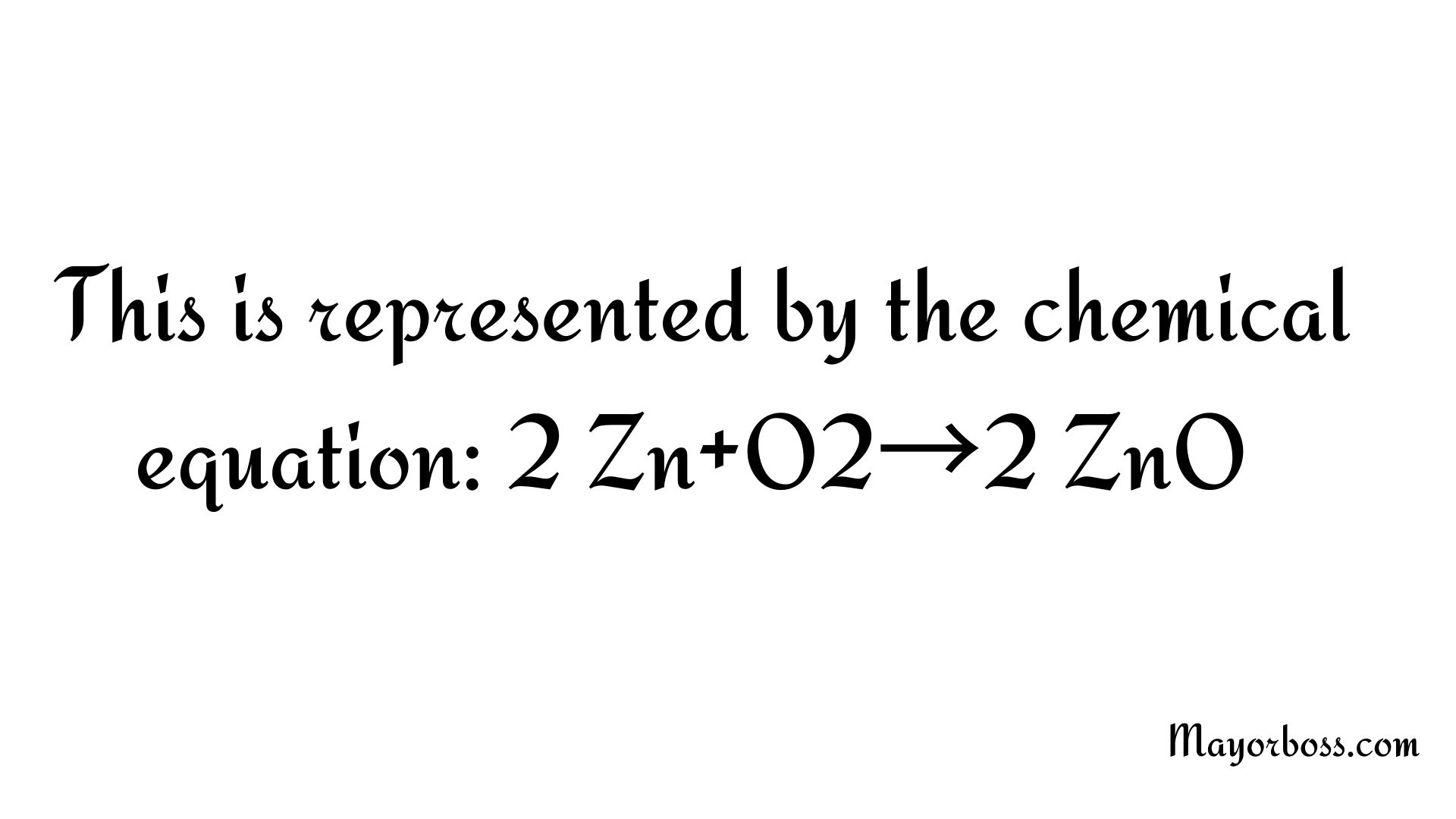
Characteristics of Zinc Oxide
- Appearance: Zinc oxide forms a white, powdery substance on the surface of zinc.
- Protective Layer: Interestingly, this layer of zinc oxide can protect the underlying zinc from further oxidation. This is why zinc is often used as a protective coating for other metals.
Practical Examples
You encounter oxidized zinc in everyday life:
- Galvanization: Zinc is used to coat steel or iron to prevent rusting. This is known as galvanization. The zinc layer oxidizes, forming zinc oxide, which then protects the steel or iron beneath from rust.
- Sunscreen: Zinc oxide is also used in sunscreens, as it provides protection from UV rays.
Conclusion
So, yes, zinc definitely oxidizes. This reaction is not only a basic chemical process but also has practical applications in various fields, from construction to skincare. The formation of zinc oxide is an essential aspect of how zinc is used and valued in different industries.
Frequently Asked Questions About Zinc Oxidation
Why Doesn’t Zinc Rust Like Iron?
You might wonder why zinc doesn’t rust like iron. This difference lies in the nature of their respective oxides:
- Zinc Oxide Formation: When zinc oxidizes, it forms zinc oxide. This oxide is quite stable and adheres well to the surface of the zinc.
- Barrier Effect: The layer of zinc oxide acts as a protective barrier. It prevents further exposure to oxygen and moisture, which are essential for continued oxidation.
In contrast, the rust formed on iron (iron oxide) doesn’t adhere as well and often flakes off, exposing more iron to oxidation. This is why iron rusts more aggressively than zinc.
Can Zinc Oxidation Be Prevented?
While zinc oxidation forms a protective layer, there might be situations where you want to minimize any oxidation. Here’s how:
- Coatings: Applying protective coatings like paint or specialized sealants can shield zinc from the air, thus reducing oxidation.
- Environmental Control: Reducing exposure to moisture and air can also slow down the oxidation process. This is more applicable in controlled environments like storage facilities.
However, it’s important to note that zinc’s natural tendency to form a protective oxide layer is often beneficial, especially in protecting other metals from corrosion.